Exploring the Origins of the Periodic Table: A Journey Through Scientific Discovery
In the vast tapestry of scientific achievement, few milestones stand as prominently as the discovery of the periodic table. This elegant arrangement of elements, organized according to their atomic properties, serves as a cornerstone of modern chemistry, guiding scientists in their exploration of the building blocks of matter.
In this journey through scientific history, we embark on a quest to unravel the origins of the periodic table, tracing its evolution from ancient philosophical musings to the meticulous experiments of pioneering chemists. Join us as we delve into the fascinating story of how humanity’s quest to understand the nature of elements led to one of the most profound discoveries in the annals of science.

Table of Contents:
- Early Chemistry and Classification Attempts
- Dmitri Mendeleev and the Periodic Table
- Discovery of the Periodic Law
- Modern Developments and Updates
- Conclusion
Early Chemistry and Classification Attempts
In the early stages of chemistry, efforts to classify elements were rooted in the quest to understand the fundamental building blocks of matter. By the year 1700, only a handful of elements had been identified and isolated, with some like copper and lead known since ancient times. As scientific methods improved, the rate of discovery accelerated, prompting chemists to seek a systematic way to organize the growing number of elements.
One notable approach was by German chemist Johann Dobereiner (1780-1849), who introduced the concept of triads in 1829. Dobereiner grouped elements into triads based on both physical and chemical properties. For instance, lithium, sodium, and potassium formed a triad where the average atomic mass of lithium and potassium closely matched that of sodium. While this triad system revealed patterns, it had limitations as not all elements could be classified in this manner.
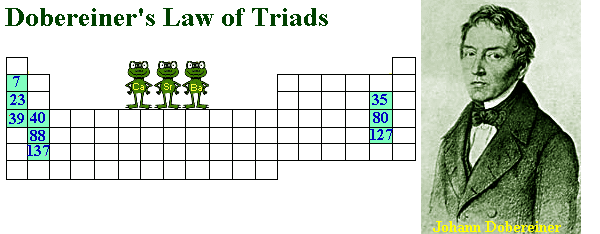
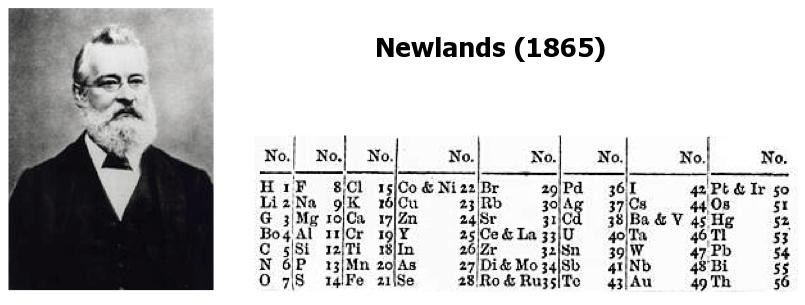
Another significant figure in early classification attempts was English chemist John Newlands (1838-1898), who observed that every eighth element, when arranged by increasing atomic mass, exhibited similar properties. He termed this relationship the “Law of Octaves.” Despite facing skepticism from his contemporaries, Newlands’ work laid the groundwork for future periodic table developments.
These early endeavors to organize elements based on properties and atomic masses set the stage for the groundbreaking work that would culminate in Dmitri Mendeleev’s creation of the modern periodic table. The journey from these initial classification attempts to Mendeleev’s revolutionary table marked a crucial evolution in our understanding of the elements and their relationships.
Stay tuned for further insights into Dmitri Mendeleev’s pivotal role in shaping the periodic table as we know it today.
Citations:
[1] https://en.wikipedia.org/wiki/History_of_chemistry
[2] https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Introductory_Chemistry_(CK-12)/06:_The_Periodic_Table/6.01:_Early_History_of_the_Periodic_Table
[3] https://tutormate.in/cbse-class-10-chemistry/early-attempts-at-the-classification-of-elements/
[4] https://www.embibe.com/exams/early-attempts-at-the-classification-of-elements/
[5] https://www.sciencemuseum.org.uk/objects-and-stories/chemistry/developing-modern-periodic-table-spirals-stars
Dmitri Mendeleev and the Periodic Table

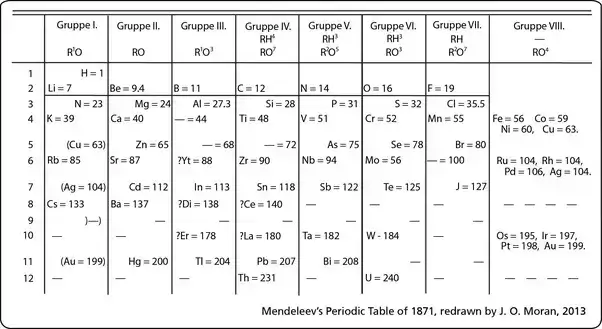
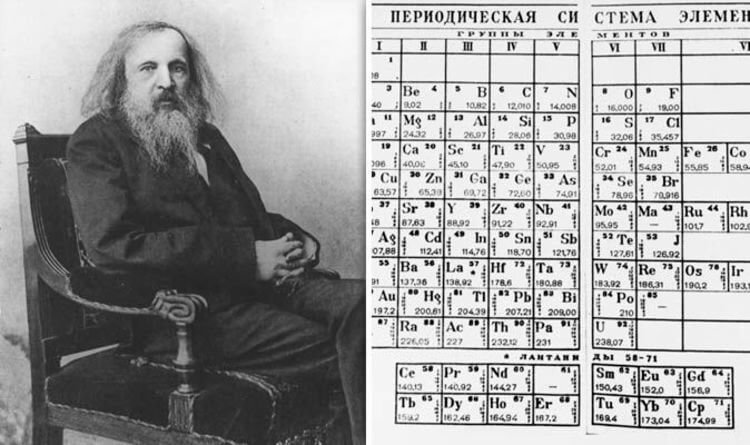
Dmitri Mendeleev, a Russian chemist born in 1834, made a groundbreaking contribution to the field of chemistry by formulating the periodic table in 1869. His methodical approach involved arranging the symbols of chemical elements based on their atomic weights, leading to the creation of the periodic table. This innovative organization grouped elements according to their properties and atomic masses, revealing patterns and regularities in their behavior.

Mendeleev’s periodic table not only provided a systematic way to categorize elements but also allowed for the prediction of undiscovered elements and their properties. By leaving gaps in his table for yet-to-be-discovered elements, Mendeleev demonstrated his foresight and confidence in the periodic law he had formulated. Remarkably, his predictions for three elements – gallium, scandium, and germanium – were later confirmed through scientific discovery, solidifying the credibility of his periodic table.
The significance of Mendeleev’s work extended beyond mere classification; it transformed the way chemists approached their discipline. His periodic table enabled scientists to predict how elements would interact with each other, the compounds they would form, and their physical properties. Mendeleev’s methodical approach and bold predictions not only revolutionized chemistry but also elevated him to a prominent position in the scientific community.
Mendeleev’s enduring legacy lies in his meticulous organization of the elements and his profound impact on the development of modern chemistry. His creation of the periodic table remains a cornerstone of chemical knowledge, reflecting his remarkable insight and contribution to the scientific world.
Citations:
[1] https://www.newscientist.com/people/dmitri-mendeleev/
[2] https://www.chemistryworld.com/features/the-father-of-the-periodic-table/3009828.article
[3] https://en.wikipedia.org/wiki/Dmitri_Mendeleev
[4] https://www.britannica.com/biography/Dmitri-Mendeleev
[5] https://www.khanacademy.org/humanities/big-history-project/stars-and-elements/knowing-stars-elements/a/dmitri-mendeleev
Discovery of the Periodic Law
The discovery of the periodic law marked a significant milestone in the development of the periodic table. Dmitri Mendeleev’s meticulous work in arranging the elements based on their atomic weights and properties led to the formulation of this fundamental law. In 1869, Mendeleev presented the first rough sketch of his table to the Russian Chemical Society, laying the groundwork for what would become a revolutionary tool in chemistry.
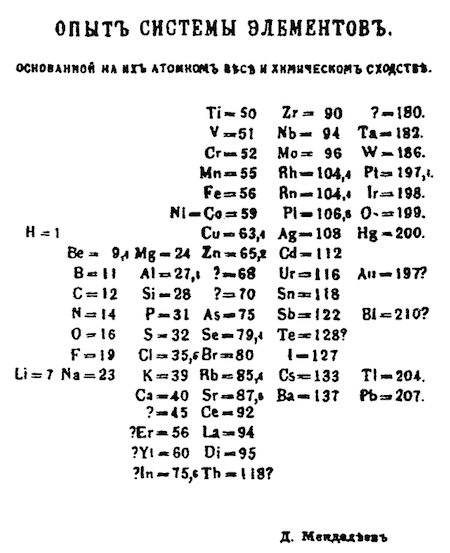
Mendeleev’s periodic table not only organized the elements but also revealed a periodic pattern in their properties. By leaving gaps for undiscovered elements and making bold revisions to known elements’ positions, Mendeleev demonstrated his conviction that the chemical elements should be viewed as a collective entity. This approach provided coherence to his table and allowed for predictions about the behavior and properties of elements that had not yet been discovered.
Despite some initial skepticism and incorrect predictions, Mendeleev’s periodic law gained credibility as new elements were discovered that matched his earlier projections. The discovery of scandium in 1879 and germanium in 1885, both exhibiting properties Mendeleev had foreseen, validated his approach and solidified his status as one of the founders of modern chemistry.
Mendeleev’s perseverance, foresight, and willingness to adjust his table based on new discoveries ultimately established the periodic law as a guiding principle in understanding the elements. His pioneering work laid the foundation for future advancements in chemistry and underscored the enduring significance of the periodic table as a cornerstone of scientific knowledge.
Citations:
[1] https://www.chemistryworld.com/features/the-father-of-the-periodic-table/3009828.article
[2] https://www.sciencemuseum.org.uk/objects-and-stories/chemistry/developing-modern-periodic-table-spirals-stars
[3] https://www.newscientist.com/people/dmitri-mendeleev/
[4] https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Introductory_Chemistry_(CK-12)/06:_The_Periodic_Table/6.01:_Early_History_of_the_Periodic_Table
[5] https://www.embibe.com/exams/early-attempts-at-the-classification-of-elements/
Modern Developments and Updates
The periodic table, a cornerstone of chemistry, saw its last update in 2016 with the addition of four new elements: nihonium (Nh), moscovium (Mc), tennessine (Ts), and oganesson (Og). These elements, numbered 113, 115, 117, and 118 respectively, completed the seventh period of the periodic table. The discovery and inclusion of these new elements not only expanded our understanding of the elements but also opened doors to potential practical applications in various fields.
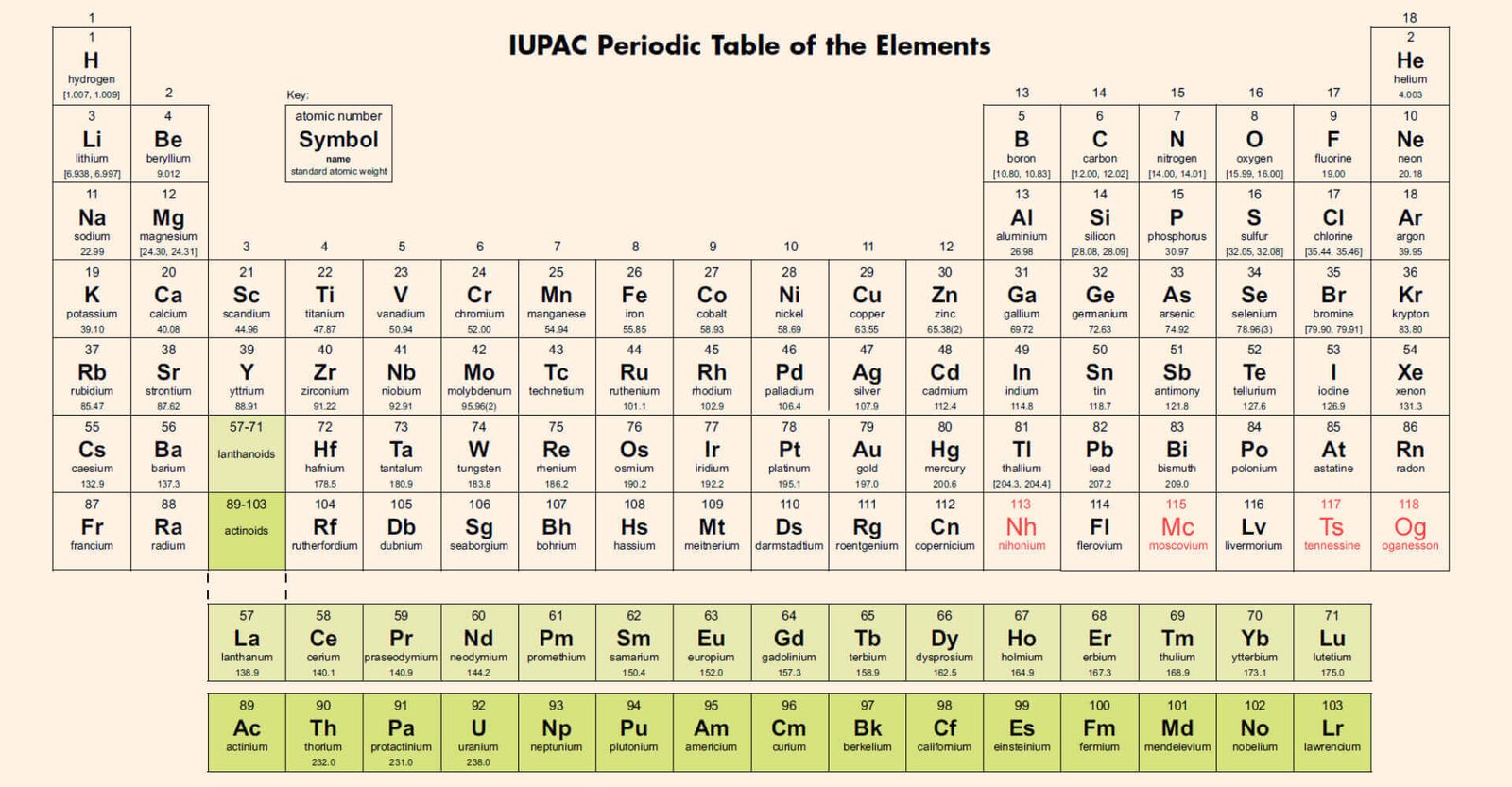
The addition of these new elements marked a significant milestone in scientific progress, showcasing the continuous evolution of our knowledge about the building blocks of matter. Each element was named after either a location or a scientist’s institution: tennessine after Tennessee, nihonium after Japan, and moscovium after Moscow. This naming convention reflects the diverse origins and contributions associated with these newly discovered elements.
Scientifically, the discovery of these heavy elements provides valuable insights into nuclear structure and stability, offering potential advancements in nuclear research and technology. Furthermore, previous heavy elements like americium and plutonium have found practical applications in smoke detectors and nuclear power sources, highlighting the real-world impact that new discoveries in chemistry can have.
As we look to the future, the periodic table remains a dynamic tool that continues to evolve with scientific advancements. The possibility of discovering more elements beyond the current table’s confines underscores the limitless nature of scientific exploration. The periodic table stands as a testament to human curiosity and ingenuity, guiding researchers towards new frontiers in chemistry and beyond.

Citations:
[1] https://letstalkscience.ca/educational-resources/stem-explained/newest-elements-on-periodic-table
[2] https://www.science.org/content/article/four-new-elements-officially-added-periodic-table
Conclusion
The discovery of the periodic table represents a pivotal moment in the history of science, transforming our understanding of the elements and their relationships. From the early classification attempts by chemists like Johann Dobereiner and John Newlands to Dmitri Mendeleev’s groundbreaking work in formulating the periodic table, the evolution of this essential tool has shaped the field of chemistry in profound ways.
Dmitri Mendeleev’s systematic organization of the elements based on atomic weights and properties not only provided a framework for classification but also enabled predictions about undiscovered elements, showcasing his visionary approach to scientific inquiry. The subsequent discovery of new elements that aligned with Mendeleev’s predictions validated the periodic law and solidified its place as a fundamental principle in chemistry.
Modern developments, such as the addition of new elements like nihonium and oganesson, highlight the ongoing evolution of the periodic table and its relevance in contemporary scientific research. As we continue to explore the frontiers of chemistry, the periodic table remains a dynamic tool that guides our understanding of the elements and their behavior.
In conclusion, the periodic table stands as a testament to human curiosity, ingenuity, and collaboration in unraveling the mysteries of the natural world. Its enduring legacy underscores the importance of scientific exploration and discovery in expanding our knowledge and shaping the future of chemistry.
Explore the wonders of chemistry in style with Chinov Display’s acrylic periodic table display featuring real elements. Perfect for enthusiasts, educators, and science lovers alike, our meticulously crafted display brings the elegance of the periodic table into your home or classroom. Elevate your space with a touch of scientific sophistication and marvel at the beauty of the elements showcased in this unique piece. Discover the perfect blend of art and science with Chinov Display’s acrylic periodic table – where knowledge meets aesthetics
Round-Cornered Periodic Table Display With Real Elements
Own a mini museum of science! This unique periodic table display features 83 real elements in clear acrylic blocks. Perfect gift for science lovers & a stunning home decor piece!✨
Ready to dive into the world of chemistry and stay updated on the latest in scientific discoveries? Subscribe to Chinov Display’s newsletter today for exclusive insights, product updates, and special offers. Join our community of science enthusiasts and be the first to know about new releases and exciting developments in the world of chemistry. Don’t miss out – subscribe now and embark on a journey of discovery with Chinov Display!


